A spectrometer is an instrument used to measure and record light waves over a part of the electromagnetic spectrum (line spectra). It determines the line spectrum of various light sources, materials and even elements. The spectrometer is also a tool for examining which parts of white light are absorbed by materials.
Done by: Ariel Lim (7), Alina Ling (8), Ankur Jain (21), Liang Cheng (22) JH404
Nature of Light: Waves
What is a wave?
A wave is a disturbance that travels from one location to another, transporting energy from one place to another as individual matter are temporarily displaced then returned to its original resting position.
Nature of light
Light acts like a wave. The wave speed of a light wave is the speed of light, and the various wavelengths of light waves are seen as different colors. The energy of a light wave is inversely proportional to its wavelength. Low energy waves have long wavelengths and thus, lower frequency; high energy light waves have short wavelengths and thus, higher frequency.
White light is made up of the 7 colors of the rainbow: red, orange, yellow, green, blue, indigo and violet. The light waves of these colors travel at different speeds and have their own wavelengths.
Process: Spectrometry
Basically, a spectrometer takes in light, separates it into its spectral components, digitizes the signal as a function of wavelength, reads it out and displays it through a computer.
Firstly, light is directed into the spectrometer through an entrance slit, diverging it.
It is then collimated by a concave mirror and directed onto a grating.
The grating disperses the spectral components of the light at slightly varying angles, which is then focused by another concave mirror and imaged onto the detector.
From the detector, data can be obtained through a software, which can be used for spectroscopic manipulations.
It is then collimated by a concave mirror and directed onto a grating.
The grating disperses the spectral components of the light at slightly varying angles, which is then focused by another concave mirror and imaged onto the detector.
From the detector, data can be obtained through a software, which can be used for spectroscopic manipulations.
*Terms:
Diverge: deflect (light rays)
Collimate: to make (light rays) parallel
Parts and functions of the spectrometer
 Slit
Slit
The slit of the spectrometer determines the angle and amount of light that enters the optical bench. Without the slit, the position of the spectral line would be spread over the range, and the wavelength cannot be calculated!Collimating mirror
The collimating mirror reflects the beams of light, making them parallel before it reaches the diffraction grating.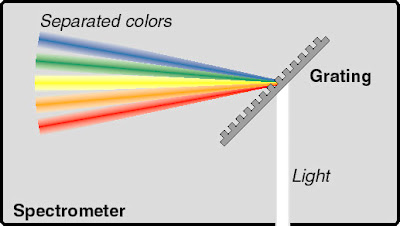 |
| Image from www.ibsenphotonics.com |
Diffraction grating/ Prism
The diffraction grating disperses the light, separating them into different wavelengths, forming the "rainbow" that can be imaged on the detector!Diffraction grating contains many lines, so that it bends light waves when it hits that grating. Different wavelengths of light bend in different ways, so it splits the light into its colors.
Some spectrometers bends light using a prism, when light reaches the prism, the speed of the light waves decrease by different amounts and thus split into different colors.
Uses of the Spectrometer
A spectrometer measure properties of light (i.e. its wavelength) over a specific portion of the electromagnetic spectrum. According to Beer's law, the amount of light that can pass through a medium is proportionate to the concentration of the light-absorbing material or solute. Using the spectrometer, we can measure the intensity of light that passes through a certain material and figure out its properties. This is process is called
spectrophotometry.

There are many uses for the spectrometer in chemistry, physics, biology and material science and astronomy. These are some common and interesting applications of the spectrometer in different fields of study:
In chemistry:
Atomic spectrometry: A technique used to study atoms and molecules. Each atom will absorb or release different amounts of energy depending on the number of electrons it has and thus emit or absorb photons in a unique pattern. By studying the light's wavelength and intensity, we can deduce the type of atom that is in a sample.
Ultraviolet Visible spectrometry: Electrons of different energy levels can absorb energy in the form of ultraviolet or visible light. Using a spectrometer, we can study the properties of light that passes through a solution and determine the concentration of absorbing species in a solution.
Infrared spectrometry:This technique deals with infrared light, which has longer wavelength and lower frequency than visible light. It is also used to study and identify chemicals and is used in both organic and non-organic chemistry.
In forensics:

With new innovation and technology comes new methods of investigating crime. Spectrometry has many uses in forensics. One example is to detect how much alcohol is present in a suspected drunk driver's blood, by identifying and analysing polymer degradation. Infrared spectrometry can also be used to study solid samples and solutions. The sample is pressed against a single crystal and infrared radiation passes through the crystal and interacts with the sample. The properties of the sample can be determined by studying the interaction between the light and the sample. Technology like this and countless others help in forensics investigation and provide evidence to solve crimes.
In astronomy:
The spectrometer is the one of the most powerful and widely used tool in astronomy,and is usually used in conjunction with a telescope. Almost all of our knowledge of the universe chemical makeup came from the spectrometer. Stars and other celestial objects emit or reflect light, a form of electromagnetic radiation. By studying the light coming from these celestial objects, we can determine or estimate many of its properties, such as distance, temperature, composition, and speed at which it is moving. When light passes through an atmosphere of gas, light is absorbed. Depending on the gasses present in an atmosphere, the wavelengths of light absorbed or emitted will be different. Dark bands in the spectrum means that energy is absorbed by an object, while bright lines usually mean the object is emitting light. Using a spectrometer, we can deduce the composition of celestial bodies, such as stars and planets. This technique is used to search for 'Earth-like' planets which may potentially sustain life. By studying the light reflected off the planet using powerful telescopes, scientists can determine the composition of the planet's atmosphere. This method proves to be useful as it allows us to study planets from light-years away without physically sending machines or humans there. As more powerful telescopes are developed, we can look further into the universe and study distant planets and stars more extensively.

(European Extremely Large Telescope (E-ELT) located in Cerro Armazones, Chile)
spectrophotometry.
There are many uses for the spectrometer in chemistry, physics, biology and material science and astronomy. These are some common and interesting applications of the spectrometer in different fields of study:
In chemistry:
Atomic spectrometry: A technique used to study atoms and molecules. Each atom will absorb or release different amounts of energy depending on the number of electrons it has and thus emit or absorb photons in a unique pattern. By studying the light's wavelength and intensity, we can deduce the type of atom that is in a sample.
Ultraviolet Visible spectrometry: Electrons of different energy levels can absorb energy in the form of ultraviolet or visible light. Using a spectrometer, we can study the properties of light that passes through a solution and determine the concentration of absorbing species in a solution.
Infrared spectrometry:This technique deals with infrared light, which has longer wavelength and lower frequency than visible light. It is also used to study and identify chemicals and is used in both organic and non-organic chemistry.
In forensics:
With new innovation and technology comes new methods of investigating crime. Spectrometry has many uses in forensics. One example is to detect how much alcohol is present in a suspected drunk driver's blood, by identifying and analysing polymer degradation. Infrared spectrometry can also be used to study solid samples and solutions. The sample is pressed against a single crystal and infrared radiation passes through the crystal and interacts with the sample. The properties of the sample can be determined by studying the interaction between the light and the sample. Technology like this and countless others help in forensics investigation and provide evidence to solve crimes.
In astronomy:
The spectrometer is the one of the most powerful and widely used tool in astronomy,and is usually used in conjunction with a telescope. Almost all of our knowledge of the universe chemical makeup came from the spectrometer. Stars and other celestial objects emit or reflect light, a form of electromagnetic radiation. By studying the light coming from these celestial objects, we can determine or estimate many of its properties, such as distance, temperature, composition, and speed at which it is moving. When light passes through an atmosphere of gas, light is absorbed. Depending on the gasses present in an atmosphere, the wavelengths of light absorbed or emitted will be different. Dark bands in the spectrum means that energy is absorbed by an object, while bright lines usually mean the object is emitting light. Using a spectrometer, we can deduce the composition of celestial bodies, such as stars and planets. This technique is used to search for 'Earth-like' planets which may potentially sustain life. By studying the light reflected off the planet using powerful telescopes, scientists can determine the composition of the planet's atmosphere. This method proves to be useful as it allows us to study planets from light-years away without physically sending machines or humans there. As more powerful telescopes are developed, we can look further into the universe and study distant planets and stars more extensively.
(European Extremely Large Telescope (E-ELT) located in Cerro Armazones, Chile)
Experiment: Making a Spectrometer
How to make a simple spectrometer:
Instructions (Make Your Own Spectrometer):
http://coolcosmos.ipac.caltech.edu/cosmic_games/spectra/makeGrating.htm
Spectrometer Diagram:

Our experiment:
We made a spectrometer and tested it out with various light sources. We used both a CD and DVD disc for 4 light sources.
Spectrometer:




Images: Spectrometer with CD and DVD with no light source
Light Sources


Light source 1: Torch Light (White light)
We shone the torch light through the 1 mm slit of the spectrometer.
 Image: Line spectrum on CD of light source 1
Image: Line spectrum on CD of light source 1
Image: Line spectrum on DVD of light source 1
Light Source 2: A flame
Next, we shone a flame through the slit of the spectrometer.
Image: Line spectrum on CD of light source 2

Image: Line spectrum on DVD of light source 2
Light source 3: Ceiling Light (Fluorescent Light Tube)
We shone light from the fluorescent light tube through the slit of the spectrometer.
 Image: Line spectrum on CD of light source 3
Image: Line spectrum on CD of light source 3 Image: Line spectrum on DVD of light source 3
Image: Line spectrum on DVD of light source 3
Light Source 4: Light Bulb (Fluorescent lamp)
Lastly, we shone the light of a fluorescent lamp through the slit of the spectrometer.
Image: Line spectrum on CD of light source 4
Image: Line spectrum on CD of light source 4
Instructions (Make Your Own Spectrometer):
http://coolcosmos.ipac.caltech.edu/cosmic_games/spectra/makeGrating.htm
Spectrometer Diagram:
Our experiment:
We made a spectrometer and tested it out with various light sources. We used both a CD and DVD disc for 4 light sources.
Spectrometer:




Images: Spectrometer with CD and DVD with no light source
Light Sources


Light source 1: Torch Light (White light)
We shone the torch light through the 1 mm slit of the spectrometer.
 Image: Line spectrum on CD of light source 1
Image: Line spectrum on CD of light source 1Image: Line spectrum on DVD of light source 1
Light Source 2: A flame
Next, we shone a flame through the slit of the spectrometer.
Image: Line spectrum on CD of light source 2

Image: Line spectrum on DVD of light source 2
Light source 3: Ceiling Light (Fluorescent Light Tube)
We shone light from the fluorescent light tube through the slit of the spectrometer.
 Image: Line spectrum on CD of light source 3
Image: Line spectrum on CD of light source 3 Image: Line spectrum on DVD of light source 3
Image: Line spectrum on DVD of light source 3 Lastly, we shone the light of a fluorescent lamp through the slit of the spectrometer.
Image: Line spectrum on CD of light source 4
Image: Line spectrum on CD of light source 4
Atom spectroscopy: Using the Spectrometer to identify Line Spectra of atoms
When the spectrometer splits white light into its colors, the spectrum of all colors of the rainbow is recorded on the photographic plate. The colors we see depend on the frequency of the light waves we see.

Image: A diagram of a simple spectrometer.
Why do some light sources have different line spectrum? Of different colors? This is due to the emission spectrum. Atoms of different elements have different electronic structures, thus, light emitted and absorbed are would vary for every element when excited. The emission spectrum of an element is the pattern of light emitted with its own characteristic pattern of frequencies. Therefore, every element had its own specific line spectrum.
Image: Line Spectrum of atoms
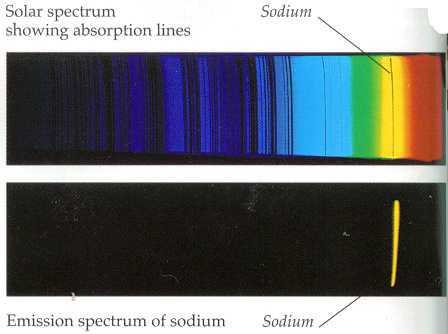
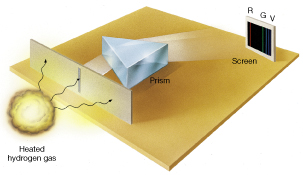
Image: A diagram of a simple spectrometer.
Why do some light sources have different line spectrum? Of different colors? This is due to the emission spectrum. Atoms of different elements have different electronic structures, thus, light emitted and absorbed are would vary for every element when excited. The emission spectrum of an element is the pattern of light emitted with its own characteristic pattern of frequencies. Therefore, every element had its own specific line spectrum.
Image: Line Spectrum of atoms
When element is heated to incandescence, it emits light that is unique to the atomic structure of the element. The light emitted is split through the spectrometer, due to the difference in frequency of light waves, thus, producing a unique line spectra of the element. Specific light frequencies give rise to sharply defined bands on the scale.
The line spectra of light emitted of elements is known as emission spectra. However, elements can absorb light too, and the absorption spectra of an element can be identified using a spectrometer. Thus, the spectrometer is able to identify both the emission and absorption spectra of elements.
Examples:
Image: Line spectra of sodium. Sodium has a very characteristic yellow band in its emission spectra and can be seen when tested with sodium vapor lamp on a spectrometer.
Image: Emission spectrum of excited (heat hydrogen gas) consists of distinct spectral lines.
Resources
Subscribe to:
Comments (Atom)







